The study of organometallic chemistry, an area that bridges inorganic and organic disciplines, has found remarkable utility in many diverse applications. Organometallic compounds themselves, which consist of metals bonded to organic groups, can teach us a great deal about fundamental bonding properties. Over the years, we have been examining the catalytic activity of the complexes of Rh, Ir and Pd. While those are expensive “noble metals” and interest globally has turned to catalysis with more “earth abundant” metals, the noble metals still have a great deal of utility because of their unique properties. Lately, we have found that the complexes we have been investigating not only have remarkable catalytic activity but also remarkable biological activity. In a most exciting discovery, we found that some of our compounds show excellent anti-microbial activity (especially against resistant bacteria such as those that cause TB and MRSA) and anti-viral activity (especially against SARS-Cov-2) while showing little to no toxicity to mammalian cells. More below!
Anti-Microbial Activity
Most antibiotics are natural products or derived from them and, so, thinking about organometallic compounds of the transition metals in that role takes a little (lot of) getting used to. We have found that many of the compounds we have been examining for their catalytic chemistry also display remarkable anti-microbial activity. Our ability to change functionality of the complexes at different sites in the molecule has led to a good understanding of structure activity relationships (SARS) and allows us to tune the complexes for different microbes. Examples of those compounds and references to our publications are shown here:
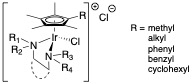
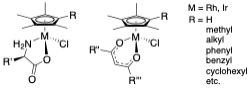
This work has been carried out in collaboration with Prof. Joe Falkinham in the VT Department of Biological Sciences. Most recent papers we have published:
1. Bernier, C. M.; DuChane, C. M.; Martinez, J. S.; Falkinham, J. O.; Merola, J. S., Synthesis, Characterization, and Antimicrobial Activity of Rh III and Ir III N-Heterocyclic Carbene Piano-Stool Complexes. American Chemical Society (ACS): 2021; Vol. 40, pp 1670-1681. DOI:10.1021/acs.organomet.1c00166
2. DuChane, C. M.; Karpin, G. W.; Ehrich, M.; Falkinham, J. O. r.; Merola, J. S.; DuChane, C. M.; Karpin, G. W.; Merola, J. S.; Ehrich, M.; Falkinham, J. O. r., Iridium piano stool complexes with activity against S. aureus and MRSA: it is past time to truly think outside of the box. Royal Society of Chemistry: 2019; Vol. 10, pp 1391-1398. DOI:10.1039/c9md00140a
3. DuChane, C. M.; Brown, L. C.; Dozier, V. S.; Merola, J. S., Synthesis, Characterization, and Antimicrobial Activity of Rh III and Ir III β-Diketonato Piano-Stool Compounds. 2018; Vol. 37, pp 530-538. DOI:10.1021/acs.organomet.7b00742
Anti-Viral Activity
Recognizing the anti-microbial activity of our complexes and being in the middle of a pandemic, the opportunity to test compounds for their anti-viral activity was too good to pass up. Thanks to our colleague James Weger-Lucarelli in the VT Department of Biomedical Sciences, we discovered that different classes of our compounds had very good anti-viral activity against Sars-CoV-2 and other viruses. Again, different functionality allows for tuning the activity of the compounds, an aspect under current investigation. The classes of complexes shown below were tested, but not all showed activity. Only the NHC and acac compounds 2 and 4 did so.
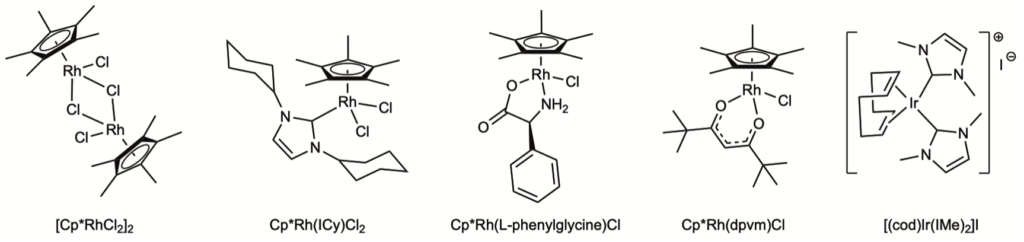
Compounds 2 and 4 in this figure show the highest activity agains Sars-CoV-2
1. Chuong, C.; DuChane, C. M.; Webb, E. M.; Rai, P.; Marano, J. M.; Bernier, C. M.; Merola, J. S.; Weger-Lucarelli, J., Noble Metal Organometallic Complexes Display Antiviral Activity against SARS-CoV-2. Viruses 2021, 13 (6). doi:10.3390/v13060980
Metal Amino Acid Chemistry for Catalysis
Some of our examination of our complexes for biological activity actually began with our investigation into amino acid complexes of metals for their catalytic activity. Amino acids provide a very inexpensive chiral ligand that allow the catalyst to produce chiral products. We have been examining several compounds for a process called asymmetric transfer hydrogenation (ATH). This process produces chiral alcohols the need for which abound in pharmaceuticals, flavors and fragrances. The latest example of our work is shown in the image below.
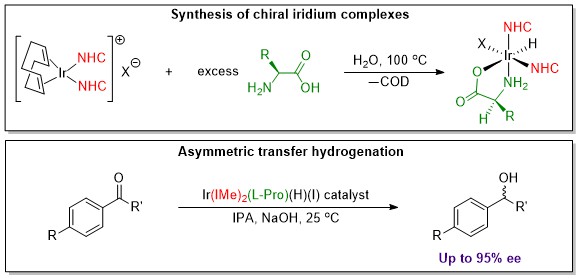
References to some of the systems we have investigated follow:
1. Bernier, C. M.; Merola, J. S., Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones. Catalysts 2021, 11 (6). doi:10.3390/catal11060671
2. Morris, D. M.; McGeagh, M.; De Pe{~n}a, D.; Merola, J. S., Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl (alkyl or aryl) cyclopentadienes (Cp∗ R), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. doi:10.1016/j.poly.2014.06.053

Professor of Chemistry and Faculty of Health Sciences at Virginia Polytechnic Institute & State University. Fellow of the American Chemical Society and the American Association for the Advancement of Science.
Education: Ph.D MIT ’78, B.S. Carnegie-Mellon ’74.
Teaching Philosophy
In 1978, fresh from a Ph.D. program in Chemistry, I joined the research laboratories at Exxon Research and Engineering Company in New Jersey. The facilities were great, the people were wonderful and the resources were what you would expect from the largest company in the world. My chemical research went extremely well and I developed a sound reputation as a researcher in catalyst chemistry. But, over nine years, there was something very much lacking and it was something that I could not easily define nor exactly articulate. Finally, that lacking solidified and I came to realize that a desire to be a teacher, especially of young minds, was strong and very much unfulfilled. I embarked upon a search for an academic position that would allow me to continue my chemical research but would also allow me be a teacher. So, in 1987, I joined the faculty of the department of Chemistry at Virginia Tech.
So, when I joined Virginia Tech, my teaching philosophy was a fairly clinical one born from shaping messages to managers: understand the level of the student and present a message that can be clearly understood by that group. Then, with each new semester, each new class, each new student, there were new things to learn and new experiences to be had. Over the course of time at Virginia Tech, I tried new things, some of which worked and some of which were abysmal failures. But the same drive that drew me to the university, also drew me to continue to experiment and push the boundaries. I was an early adopter of a lot of different technologies in the classroom as part of my search to find the best way to shape those young minds entrusted to me. I learned that technology is a useful tool, but still only a tool, not a great thing in and of itself. PowerPoint was a wonderful way of organizing and presenting information. It was also a great way to overload the students and cover material way too fast for student comprehension. In all these endeavors, I learned and shared what I learned through presentations and publications. I was honored that one of these publication was chosen by Microsoft and placed on a DVD of best practices at that time.
What I would call my purely clinical view of teaching took a very different turn after my own children (4 of them) became of college age beginning about ten years ago. All of them went to different schools so I saw things from a very different and, frankly, a very visceral point of view. My philosophy, while developing in an evolutionary way over my first 15 years, took a revolutionary leap: teach your students as you would want your own children to be taught. That involves a lot more than just delivering a “clear message”. It brings in the need to be patient, to try and understand all of the things that may be going on in the student’s life. It is easy to believe that the “good students” will get the material and for those who don’t, well, they clearly didn’t have what it takes. It involves trying as much as possible to work with individual students, to find their needs and to work with the student to address them. This is not easy to do with classes of 200-500 students and it requires a large time commitment outside the lecture hall, but it IS possible. When I viewed the students as I would want my own children to be viewed, then things “clicked”. The time spent outside of the classroom was not a bother, but a real joy. Counseling sessions with the students who performed poorly on exams was no longer a perfunctory email telling them to get into shape, but times to get to know my students at a deeper level, often being able to construct successful strategies for them to succeed.
Even though I have been at it for over thirty years now my philosophy of teaching is still evolving. If I were to be so lucky (and so unusual) as to be teaching thirty years from now, maybe by then, I will have gotten it (almost) right.
References
I am very much a technology geek, but I also recognize that technology is just another tool to be used and it is not the panacea that will solve all problems associated with teaching and learning. In fact, when used poorly or improperly, technology can be a huge barrier to effective teaching. A little over ten years ago, about ten years into my teaching career, I was an early adopter of the internet and the web. Those activities seem extremely primitive by today’s standards, but for the time they were on the cutting edge and the lessons learned are still applicable today. If you are interested in reading about those “archaic” uses of technology, you can visit the Technology Source Archives at: http://horizon.unc.edu/projects/monograph/CD/Science_Mathematics/Merola.html
This article was included in a special CD distributed by Microsoft entitled “Technology Tools for Today’s Campuses”. The CD is out of print, but the contents can be found here: http://horizon.unc.edu/projects/monograph/CD/
My specific article is at: http://horizon.unc.edu/projects/monograph/CD/Science_Mathematics/Merola.asp
An important dimension of teaching that all scientists should embrace is communicating science to the general public so that the “mystery” shrouding scientific research is dispelled. To that end, I have made myself available to answer questions of a chemical nature for broad audiences. For Scientific American “Ask the Experts”, I have contributed the following four articles:
- How do air bags work?
- What is chemiluminescence?
- Why isn’t the dual wave/particle nature of the quantum mechanical world present in the macroscopic world?
Since 1997, I have been a regular, invited contributor to the AAAS Radio Program “Science Update”. Over this period, I have had 24 different interviews that have aired on this nationally syndicated program. For sample contributions, see the following:
- “Where Do Heavy Elements Come From”
- “Why Is Snow White When Water and Ice Are Clear”
You can listen to the program by clicking on the “listen” button. In many of the programs the narrator refers to me as their “favorite chemist.”
Dr. Joseph Merola's Google Scholar:
https://scholar.google.com/citations?user=lfugPyUAAAAJ&hl=en&oi=ao
A Sampling of Publications Over the Years
Chuong, C.; DuChane, C. M.; Webb, E. M.; Rai, P.; Marano, J. M.; Bernier, C. M.; Merola, J. S.; Weger-Lucarelli, J., Noble Metal Organometallic Complexes Display Antiviral Activity against SARS-CoV-2. Viruses 2021, 13 (6). doi:10.3390/v13060980
Bernier, C. M.; Merola, J. S., Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones. Catalysts 2021, 11 (6). doi:10.3390/catal11060671
Bernier, C. M.; DuChane, C. M.; Martinez, J. S.; Falkinham, J. O.; Merola, J. S., Synthesis, Characterization, and Antimicrobial Activity of RhIII and IrIII N-Heterocyclic Carbene Piano-Stool Complexes. Organometallics 2021, 40 (11), 1670-1681. doi:10.1021/acs.organomet.1c00166
DuChane, C. M.; Karpin, G. W.; Ehrich, M.; Falkinham, J. O.; Merola, J. S., Iridium piano stool complexes with activity against S. aureus and MRSA: it is past time to truly think outside of the box. MedChemComm 2019, 10 (8), 1391-1398. doi:10.1039/c9md00140a
Brown, L. C.; Ressegue, E.; Merola, J. S., Rapid Access to Derivatized, Dimeric, Ring-Substituted Dichloro(cyclopentadienyl)rhodium(III) and Iridium(III) Complexes. Organometallics 2016, 35 (24), 4014-4022. doi:10.1021/acs.organomet.6b00580
Karpin, G. W.; Morris, D. M.; Ngo, M. T.; Merola, J. S.; Falkinham, J. O., III, Transition metal diamine complexes with antimicrobial activity against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA). MedChemComm 2015, 6 (8), 1471-1478. doi:10.1039/C5MD00228A
Morris, D. M.; McGeagh, M.; De Pea, D.; Merola, J. S., Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl (alkyl or aryl) cyclopentadienes (Cp∗R), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. doi:10.1016/j.poly.2014.06.053
Merola, J. S.; Knorr, J. R., Synthesis and reaction chemistry of boryliridium hydride complexes formed by oxidative addition of catecholborane to iridium (I): Lessons for metal-catalyzed hydroboration. Journal of Organometallic Chemistry 2014, 750, 86–97. doi:10.1016/j.jorganchem.2013.10.049
For a complete list of publications see: MerolaCV_2022
Publications with Unusual Crystal Structures
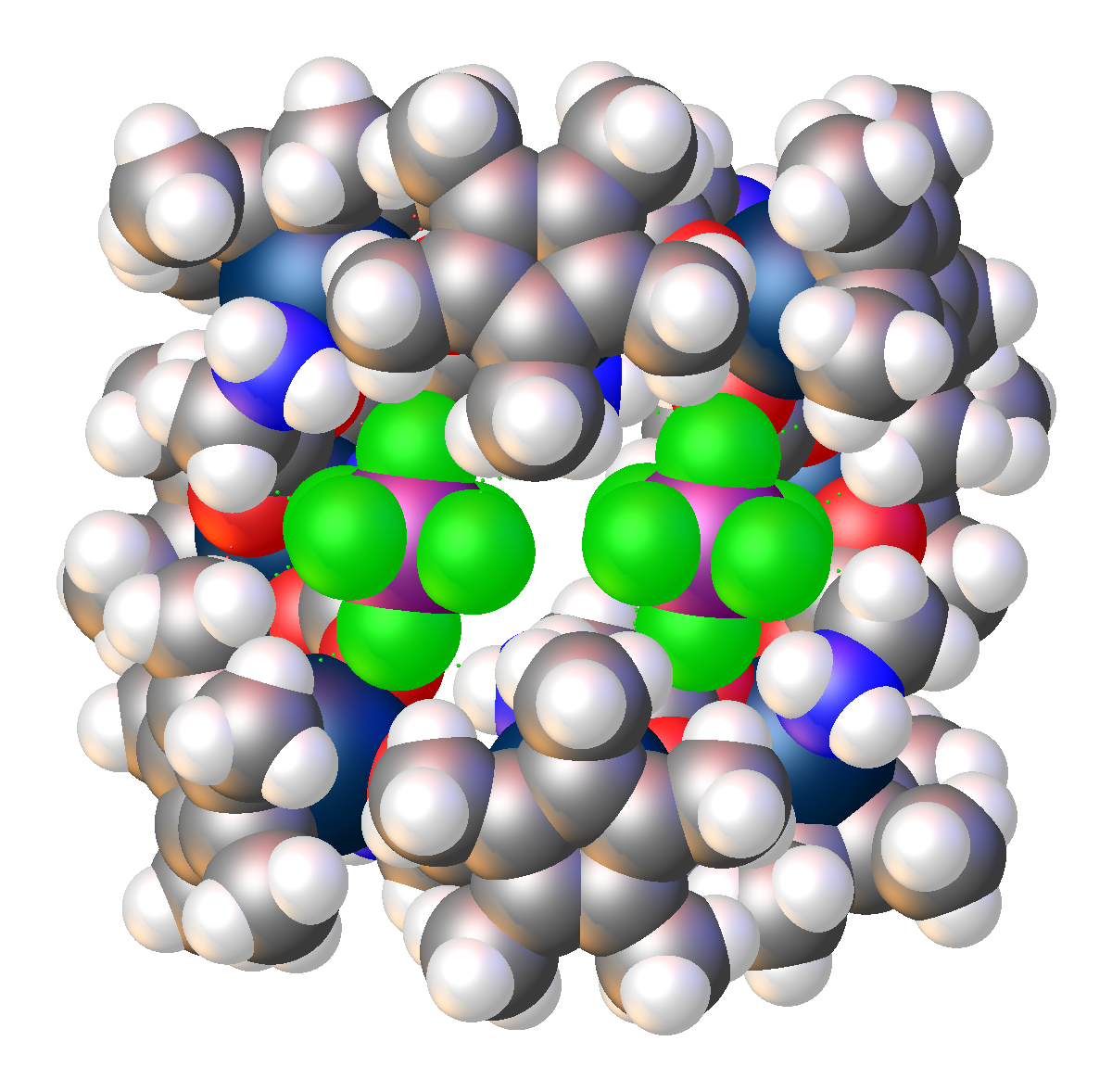
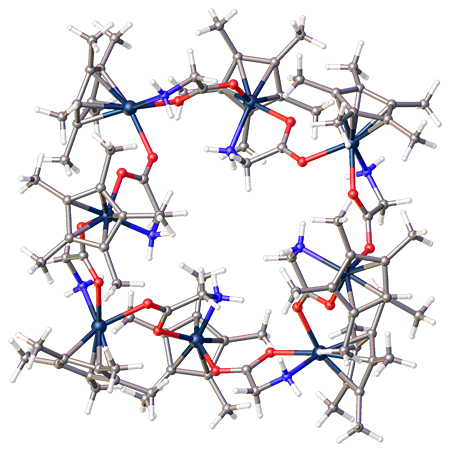
The crystal structure of an octametallic iridium piano-stool cluster can be found in 1. Morris, D. M.; Merola, J. S., Octametallic Cluster of Cp*Ir(glycinato) Cations. ACS Omega 2019, 4 (26), 22126-22132. doi:10.1021/acsomega.9b03267 Link to paper This structure was featured as one of 2020’s “Molecules of the Week” by the American Chemical Society. This article can be found hereLink to paper

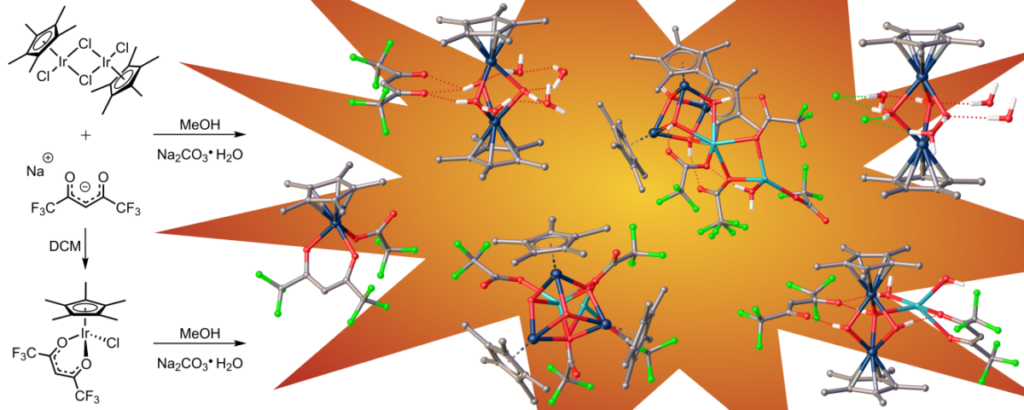
While cubane-type structures in inorganic chemistry are not novel, the ones with iridium and oxygen found in this paper are. There are additional unusual structures in this paper as well. 1. DuChane, C. M.; Merola, J. S., Hexafluoroacetylacetonate (hfac) as ligand for pentamethylcyclopentadienyl (Cp*) rhodium and iridium complexes: Some surprising results, including an Ir3Na1O4 cubane structure. Journal of Organometallic Chemistry 2020, 929, 121552. doi:10.1016/j.jorganchem.2020.121552Link to paper
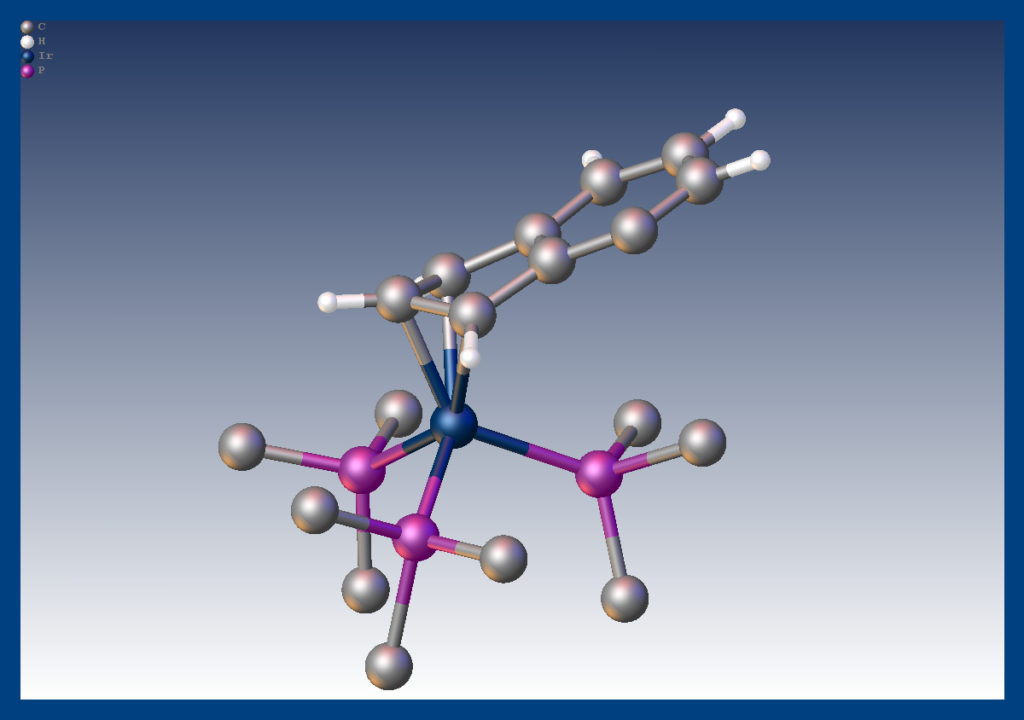
It had long been postulated that the greater reactivity of indenyl metal complexes compared with their cylopentadienyl analogs was due to the propensity of the indenyl ligand to switch from η5 to η3 coordination. We succeeded in isolating an η3 complex of indenyl with iridium showing it is a feasible coordination mode for that ligand. Merola, J. S.; Kacmarcik, R. T.; Van Engen, D., The. eta. 5 to. eta. 3 conversion in indenyliridium complexes. Journal of the American Chemical Society 1986, 108 (2), 329–331. doi:10.1021/ja00262a042 Link to paper
Dr. Joseph Merola, Principal Investigator

Professor of Chemistry and Faculty of Health Sciences at Virginia Polytechnic Institute State University. Fellow of the American Chemical Society and the American Association for the Advancement of Science.
Education: Ph.D MIT ’78, B.S. Carnegie-Mellon ’74.
Current Members
Jorge Nasser, Ph.D. Research Associates
Mike Berg, Ph.D. Instructor and researcher
Alec Wagner, Ph.D. Instructor and researcher
Sharon Molnar, Ph.D. collaborator from WVSU
Hannah Beaver, undergraduate researcher
Mattius Felicioni, undergraduate researcher
Brendan Gallagher, undergraduate researcher
Ashley James, undergraduate researcher
Stefanie Kerenyi, undergraduate researcher
Sophia Trozzo, undergraduate researcher
Charles Vasser, undergraduate researcher
Alumni
The success of the Merola research program rests solely on the shoulders of the students who have been a part of the group: summer students, undergraduate research students and graduate students. Here are highlights from some of the students who have passed through the group and what they are doing now.
Graduate Students
Chad Bernier
Loren Brown
Christine Duchane
David Hobart
George Karpin
Shannon R. Chiles
Robert Clark
Beth Dodson
Joy Frazier
Marion A. Franks
Arthur Grieb
Carl Heltzel
David Hobart
Lisa Huff
Joseph Knorr
Folami T. Ladipo
Trang Le
Kelly Matthews
David Morris
Robert Pafford
Christopher P. Roy
Hank Selnau
Research Associates
Michael Berg, Senior Instructor of Chemistry, Virginia Tech
Alec Wagner, Instructor of Chemistry, Virginia Tech
Undergraduate Students
BillyClarke
Cole Martin
Andy An
Zoe Stuart
Savannah Phillips
Justin Martinez
Billy Owen
Gina Asche
Nick Barker
Joel Bielski
Pauline M. Boyer
Paul J. Chirik
Debra Driscoll
Julie Hoher
Margaret S. Richards
Mel Ritchie
Ellen Slusher
Gita Srinivasan
Gina Yanochko
Alex Collado
Caitlin Masterman
Alex Mai
Mike Peysakhovich
Tefsit Bekele
Tsion Bililign
Steve Yun
( A number of undergraduates are missing from this list? If you remember our additional ones, please let me know!)
Summer Visitors
John Clancy (IAESTE intern)
Rebecca Randall (IAESTE intern)
Visiting Scientists
M. Kooti (Iran)
Sharon Molnar, Professor of Chemistry, West Virginia State University
Technicians
Bill Bradbury
Frank E. Anderson III
